
Amos Miller and the Regulation of Raw Milk
- April 25, 2024
- Emily Stone
Discussions among legislators, public health officials, and consumers persist across the United States as the debate over raw milk sales continues....

The Feed: Volume 2, Issue 8
- April 24, 2024
- Ramie Parsons
The Feed newsletter highlights recent legal developments affecting agriculture, with issues released twice a month. Click below to sign up for...

Federal Bills Propose Changes to the H-2A Program
- April 18, 2024
- Samantha Capaldo
In 2022, agriculture and food related jobs accounted for approximately ten percent of employment in the United States. H-2A workers play...

FWS Streamline ESA Voluntary Conservation Programs
- April 16, 2024
- Brigit Rollins
The United States Fish and Wildlife Service (“FWS”) have issued a final rule to update the implementing regulations for Section 10...

The Feed: Volume 2, Issue 7
- April 16, 2024
- Ramie Parsons
The Feed newsletter highlights recent legal developments affecting agriculture, with issues released twice a month. Click below to sign up for...

Ag, water and environmental law experts convene at University of Nevada, Reno
- April 12, 2024
- Ramie Parsons
Ag, water and environmental law experts convene at University of Nevada, Reno National Ag Law Center presents 2nd Annual Western Agricultural...

Update on Proposed Food Additive Bans
- April 11, 2024
- Emily Stone
In October 2023, California passed the California Food Safety Act becoming the first state to ban the manufacturing, distribution and sale...

The NALC Quarterly Newsletter: 1Q24
- April 5, 2024
- Ramie Parsons
The National Agricultural Law Center compiles and publishes a quarterly newsletter highlighting recent and upcoming events and resources. The newsletter...

SEC Rule and California Laws on Climate-Related Disclosures Face Legal Challenges
- April 4, 2024
- Samantha Capaldo
UPDATE: On April 4, 2024, the SEC announced a voluntary stay on the climate-related disclosure rule. This voluntary stay is intended to...

Farmers File Suit Over PFAS Contamination
- April 2, 2024
- Brigit Rollins
In February 2024, a group of Texas farmers filed a lawsuit against Synagro Technologies, Inc. (“Synagro”) for manufacturing and distributing a...

March Monthly Roundup
- April 2, 2024
- Ramie Parsons
March Roundup As March has ended, the staff at the National Agricultural Law Center continue to bring trusted research and information...

The Feed: Volume 2, Issue 6
- March 28, 2024
- Ramie Parsons
The Feed newsletter highlights recent legal developments affecting agriculture, with issues released twice a month. Click below to sign up for...

USDA Finalizes Voluntary “Product of USA” Rule
- March 28, 2024
- Emily Stone
In March 2024, the United States Department of Agriculture (USDA) finalized its rule regarding the voluntary use of the labeling terms...
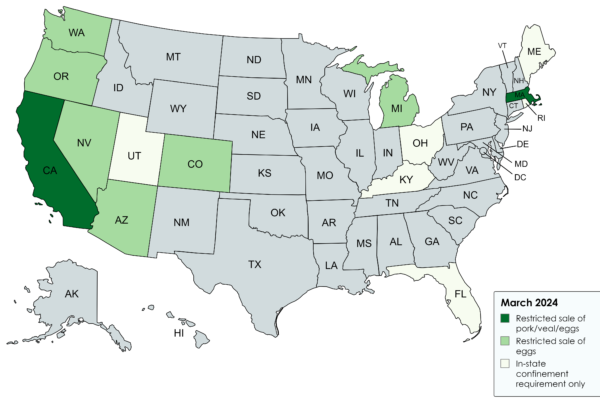
Farm Animal Housing in 2024: Laws, Proposals and Challenges
- March 26, 2024
- Elizabeth
In 2018, California passed Proposition 12, a high-profile ballot initiative regulating the production and sale of many veal, egg, and pork...

The SEC Finalizes Rule on Required Climate-Related Disclosures
- March 21, 2024
- Samantha Capaldo
UPDATE: On April 4, 2024, the SEC announced a voluntary stay on the climate-related disclosure rule. This voluntary stay is intended to...

Limits on Land Acquisitions: Three States Amend Their Foreign Ownership Laws
- March 19, 2024
- Elizabeth
Since January 2024, the majority of states have proposed at least one piece of legislation to prohibit or restrict foreign investments...

The Feed: Volume 2, Issue 5
- March 15, 2024
- Ramie Parsons
The Feed newsletter highlights recent legal developments affecting agriculture, with issues released twice a month. Click below to sign up for...

Plaintiffs Claim Cranberry Bog Not Exempt from CWA Regulation
- March 14, 2024
- Brigit Rollins
On February 28, 2024, the Corte Oreilles Lakes Association together with the Lac Courte Oreilles Band of the Lake Superior Chippewa...
France takes up plant protein labeling and cell-cultured meat
- March 12, 2024
- Emily Stone
The conversation over how to label food products made from plant-proteins and cell-cultured meat is heating up in Europe. Like the...

EPA Delays Rescinding Gasoline Volatility Waivers Until 2025
- March 7, 2024
- Samantha Capaldo
In 2023, approximately forty percent of corn produced in the United States (“U.S.”) was used for ethanol production. The top three...
